The Medicines and Healthcare products Regulatory Agency and the Maritime and Coastguard Agency are jointly issuing a warning that imported life rafts may contain unlicensed medicines.
Suppliers of inflatable life rafts in the UK are being asked to check the origins of the products they sell.
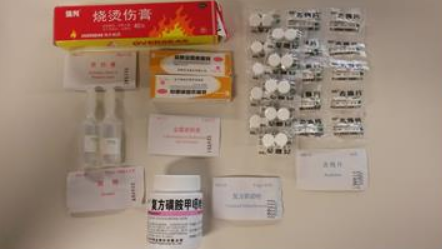
This is because medical kits containing unauthorised imported medicines and medical devices may have been supplied inside inflatable life rafts to ships, fishing vessels, small commercial vessels and pleasure boats.
The Maritime and Coastguard Agency has now issued a safety bulletin about the issue.
Suppliers of imported life rafts are asked to open at least one life raft from each non-UK supplier they deal with and check that the medical kit only contains medicine and medical devices authorised for use in the UK.
This follows an inspection by the Medicines and Healthcare products Regulatory Agency (MHRA).
It found that some UK service stations, manufacturers and distributors of life rafts had been importing and supplying medical kits that contain Chinese medicines and medical devices. These are not authorised for use in the UK.
If the kit contains unauthorised medicines or medical devices it should be disposed of by an approved pharmaceutical waste contractor.
Owners of life rafts who have any doubt as to the compliance of the medical kits within their life rafts should contact the supplier or service station who serviced, sold or rented the life raft to them for advice.
The MHRA Director of Inspection, Enforcement and Standards, Gerald Heddell, said: “It is important that the medical kits contained in these life rafts only contain medicines and medical devices authorised for use in the UK. Whilst they are only likely to be taken in the rare event of a life raft being deployed in an emergency it is important they are of the right quality and standards.”
He continued: “If the kit does not contain authorised medicines or medical devices they should be returned to the supplier for disposal at an approved pharmaceutical waste disposal site. Anyone who has used medicines or medical devices originating from a medical kit on an inflatable life raft and who is concerned should contact their healthcare professional for advice.”
Medicines supplied for use within the UK must be approved by either the MHRA or the European Medicines Agency.
Recall on Kannad SAFELINK EPIRBs
Kannad Marine has instigated a global recall programme following issues with the company's SAFELINK Manual and Auto GPS EPIRBs.
10 Top innovations in the history of sailing
From celestial navigation to GPS technology, these sailing innovations have changed the way we take to the sea. And we…
Pictures: The next generation lifeboat from Berthon
Berthon has announced there is growing overseas interest in the new 14 metre search and rescue vessel.